The Bolt-on Bioreactor design
|
The Bolt-on Bioreactor is an automated and efficient perfusion bioreactor for the culture of adherent cells. The design of the BoB is the result of a thorough understanding of the challenges faced by cell culturers compared to features of current cell culture systems, and the contribution of the Market through an Open Design initiative carried out for half a year among thousands of persons involved in adherent cell culture. |
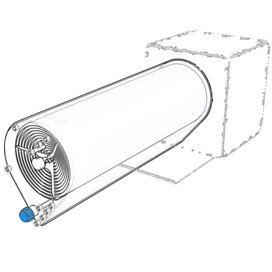 |
Following, you will find a detailed description of the Bolt-on Bioreactor design and the explanation of the major features of this perfusion bioreactor. The different features of the BoB are described following the same order in wich the user will encounter each of them during system set-up:
|
Cell culture chamber |
 |
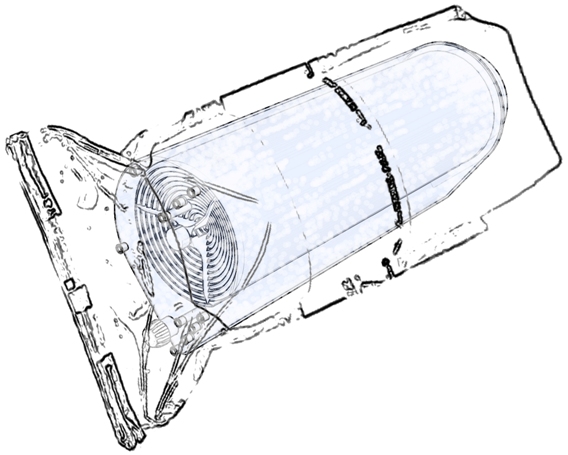
The single-use culture chamber is supplied sterile, gamma irradiated, within a double plastic bag. Adhesion surface for cells is a continuous and smooth membrane with the shape of a rolled membrane where gentle and constant recirculation and complete, uniform distribution of the culture medium is ensured by the liquid flow path resulting from the rotatory movement of the rolled membrane. Cell culture area for the first BoB culture chamber is 25,000 cm2. A 5,000 cm2 version and a 50,000 cm2 version of the the product are to follow shortly.
|
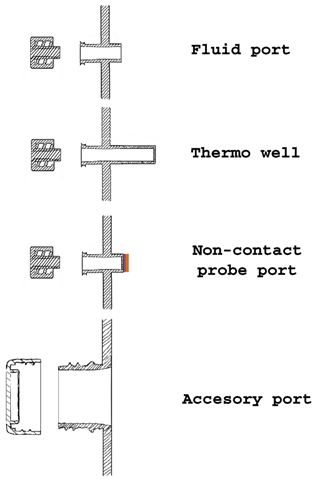
Due to the development of a purposely designed agitation system (see the solution to Challenge #3 for details) the culture chamber is a static and fully contained space with a continuous wall interrupted only by the fluid transfer ports. Apart from fluid transfer ports, the culture chamber is provided with ports for non-contact probes and additional ports for user-defined purposes. All ports, except for the accessory standard thread port for user-defined purposes, are standard female luer ports, both for probes and fluid transfer connections. Except for custom orders, all ports are provided with standard protecting caps.
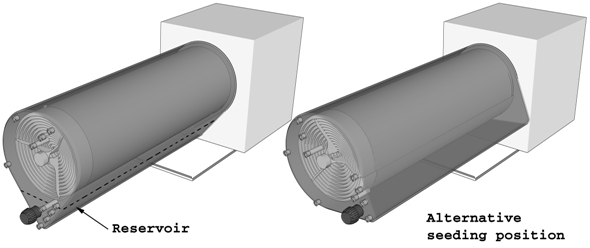
A culture medium reservoir at the bottom of the culture chamber serves various purposes: i) acts as a buffering system to mitigate sudden changes in culture medium composition and temperature; ii) provides a medium reservoir when batch mode is preferred over perfusion mode culture; and iii) stabilizes process parameters readings for proper monitoring and control. However, when a reduced volume of cell suspension is available at the initial seeding stage, the alternative seeding position of the culture chamber allows for an equally efficient seeding step previous to the continuous, perfused culture process. |
 |
Culture medium flow
Perfusion cell culture is presumed to be the default option for most users, therefore and inlet port for culture medium and the corresponding outlet port for collecting outgoing used medium are expected to operate simultaneously. Since outgoing flow is set to be larger than ingoing flow, location of the outlet port limits the maximum culture medium level within the culture chamber thus allowing for an optimum usage of the system’s capacity. The medium reservoir at the bottom of the culture chamber combined with the liquid flow path within the rolled membrane-shaped adhesion surface, provides an efficient and gentle liquid mixing and distribution system. A further Harvest port at the bottom of the culture chamber guarantees optimal recovery of the product at the end of the process and complete emptying of the culture chamber during washing steps.
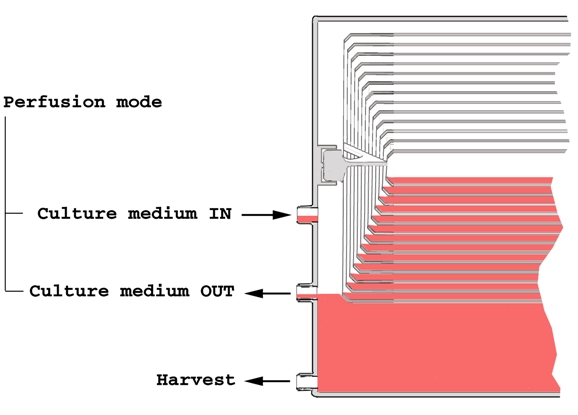
While excreted biopharmaceuticals can be continuously collected during perfusion operation via the outlet port, harvest can be performed by suction using peristaltic pumps or by pressurizing the culture chamber by pumping circulating gas mix while blocking gas exhaust. Three additional ports are available for alternative, user-defined, set-ups.
Default liquid transfer ports are standard female luer connectors, although an accessory standard thread port is available for particular needs. Since liquid transfer ports give open access to the interior of the culture chamber, connection to these ports should occur within clean environment laboratories to avoid contamination of the culture. Alternatively, quick sterile connectors can be attached to liquid transfer ports to provide safe connection in non-controlled environments. Sampling can proceed through a septum installed in the standard threaded port, or through one of the spare luer ports.
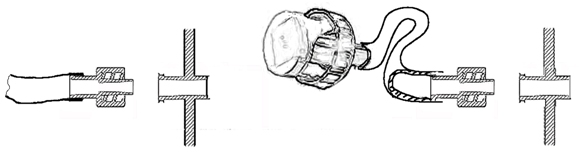
Using transfer cap-terminated tubing, culture medium flasks can be connected directly to the culture chamber. For extended cultures where additional flasks must be connected over time, tubing with multiple connections can be used. When integrated peristaltic pumps are used, the control unit will regulate culture medium replacement rate to meet programmed requirements.
Gas flow
The appropriate gas mixture enters the culture chamber through a sterilizing filter directly connected to the culture chamber via female luer connection. Exhaust gas exits the culture chamber via the gas outlet port. The outlet port is provided with a heated venting filter attached to the culture chamber through luer connection. Heating the filter prevents blockage of the filter due to condensation on the filtering membrane.
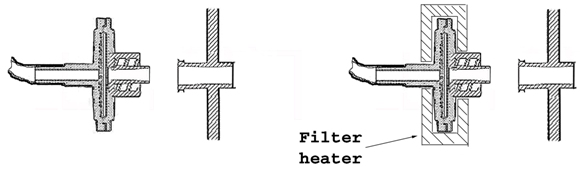
A pressurized gas mixture source at regulated pressure can be connected to the culture chamber through the gas inlet filter directly or via the control unit. When the gas mix source is connected to the control unit, gas replacement within the culture chamber will occur continuously at a very low rate to ensure even gas composition throughout the process. The control unit will also heat the flowing gas in response to gas phase temperature readings below the set-point, within the chamber. Variations to the flow speed will occur in response to CO2 concentration decays. Since gas inlet and outlet ports are open to the culture chamber interior, in order to avoid contamination filter connection must take place in controlled environment atmosphere unless the culture chamber is provided with the filters in place.
User-defined probe
Three spare ports are provided for particular, user-defined requirements. These ports provide open access to the interior of the culture chamber and therefore care should be taken to avoid contamination when manipulating these ports.
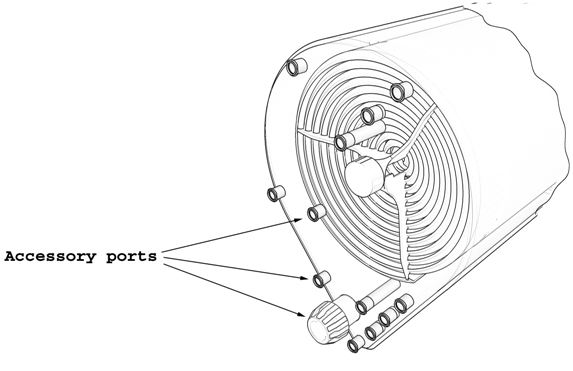
One of these spare ports is a standard thread port located below liquid level and designed to provide additional access to the culture medium for user-defined measurements. The chosen probe should be provided by the user and sterility of the probe ensured before attaching the probe to the system.
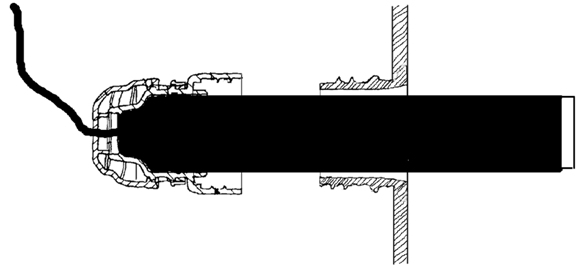
The following elements of the system do not require for their installation access to the interior of the culture chamber. Since containment of the culture chamber is not affected, manipulation of the system does not require controlled environment laboratories.
Control unit
The rotating cell adhesion surface in the culture chamber is engaged with the agitation system in the control unit by means of a short turn of the chamber. By default, the culture chamber is bolted on the control unit on the culture position. Alternatively, as mentioned in the Culture chamber section, for small volume seeding, the culture chamber can be bolted on the control unit in the seeding position.
The revolutionary agitation system developed for the Bolt-on Bioreactor provides a complete physical separation of the spaces occupied by the culture chamber and the mechanical elements within the control unit. This feature allows for the control unit to be segregated in a separated room, therefore dramatically reducing contamination risk and the space necessary for the cell culture laboratory, and allowing for technical maintenance of the control unit from outside the cell culture laboratory.

The control unit takes care, via programming or manual operation, of rotatory speed and direction of the cell adhesion surface within the culture chamber. Special programs can be used during seeding step to ensure proper cell attachment before proceeding with the perfusion or batch culture.
The control unit also controls the thermostatic jacket, the gas mixture heating and impulsion system and medium replacement rate.
pH, CO2 and Dissolved Oxygen control
Non-contact probes are used for the measurement of pH, CO2 and Dissolved Oxygen (DO). Probes are connected to the culture chamber via standard luer connectors and into a pouch where a signal proportional to the concentration of the analyte is sent to the transmitter via optic fiber. Single-use, specific sensing elements included in the interior of the culture chamber are responsible for generating the signal transmitted via the re-usable optic fiber probe.
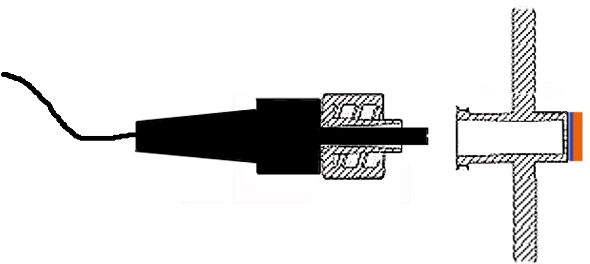
When the signal transmitter is integrated with the control unit, gas flow and medium replacement rates are adjusted based on the value of process parameters.
Temperature control
Temperature control is achieved by the combined effect of four elements: i) a non-contact probe that measures the temperature of the gaseous phase; ii) a non-contact probe that measures the temperature of the liquid phase; iii) a heating jacket responding to the temperature of the culture medium and; iii) a gas mix heating element responding to the temperature of the gaseous phase. All four elements are controlled by the control unit. The simultaneous control of gaseous and liquid phase temperature ensures optimum temperature conditions for the cells and eliminates temperature variations between liquid and gaseous phases.
Temperature sensors are non-contact probes located within thermo-wells and connected to the culture chamber via standard luer ports.
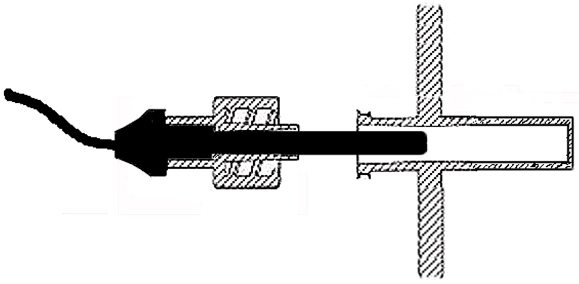
The heating jacket completely surrounds the culture chamber for an effective temperature control and protects the cells and culture medium from the potentially deleterious effect of light.
The following video summarizes the most relevant elements of the Bolt-on Bioreactor.
© Copyright 2014 - 2019, The BoB project
